Medical Research
World Leader in Medical Research
Our products and research results are used in hospitals and labs all over the world and thus contribute to greater global health.
How are medical isotopes produced?
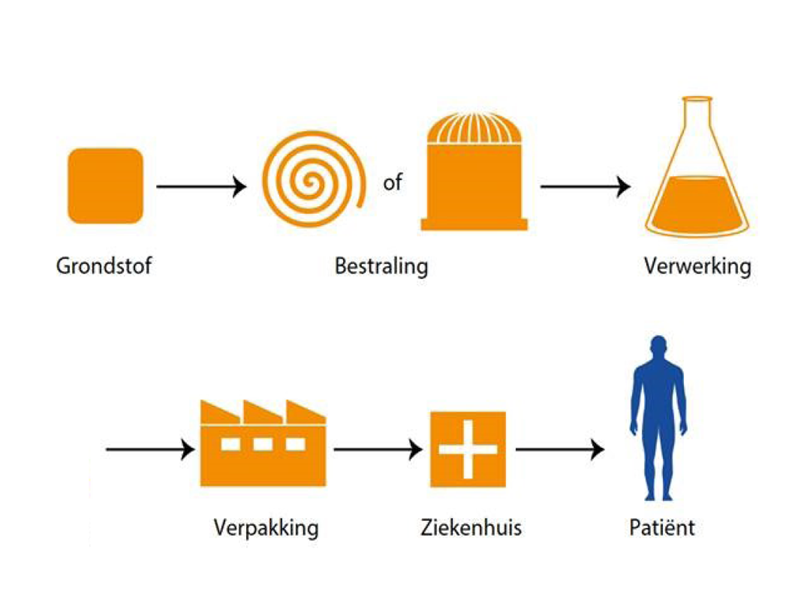
From radioisotope to radiopharmaceutical, i.e. nuclear medicine. To explain the impact of NRG’s medical research and how we contribute to a healthier society, it is important to understand how medical isotopes are produced.
First, the primary material is irradiated in a reactor or accelerator. In the process, the base material becomes radioactive. At this stage, a radioisotope (RI) is produced. The irradiated material is then purified and after processing in several steps, the radiochemical (RC) is produced. This forms the basic material for substances used in nuclear medicine. This material is then used to develop a medical product. That medical product – a radiopharmaceutical (RP) – consists of a chemical bond with the medical isotope as one of its components. This entire process is carried out under the strictest quality controls. The radiopharmaceutical brings the medical isotope as close to the diseased cells as possible. The radiation in the isotope destroys those sick cells, causing as little damage as possible to healthy cells. We have created a new generation of treatments with great potential particularly for cancer.
Our research areas
Our research focuses on developing and modifying the production chain for existing and new radiopharmaceutical products. Three areas are important here:
Processing technology: developing knowledge and infrastructure dedicated to the chemistry and purification of irradiated materials. The conversion of the radioisotope into the radiochemical (RC) or radiopharmaceutical (RP).
Irradiation technology: maintaining the level of knowledge and infrastructure for irradiation and knowing how to produce a radioisotope from the primary material under the right radiation conditions.
Handling production and R&D facilities for radiopharmaceutical drugs. This requires maintaining knowledge in nuclear science (safety, radiation, waste) on the one hand, and in the medical field (Good Manufacturing Practices, GMP) on the other. Such knowledge is necessary if we want to be ready for the products of the future.
GMP is a quality control system. The quality and purity of the medicinal products administered can only be safeguarded if the entire production process is regulated and controlled.
Hospitals carry out research into the development of new radiopharmaceutical drugs. For clinical trials, small amounts of this radiopharmaceutical substance are needed. The substance is not yet available, so a production process needs to be developed for it. We do this in the FIELD-LAB. Conducting clinical trials and developing a drug is time consuming and expensive. FIELD-LAB is dedicated to accelerating the development of new radiopharmaceutical drugs. NRG works in close collaboration with the academic medical centres and other partners to ensure that new medicinal products will also be produced and supplied in the future
Read more
Contact

Sander de Groot
Sr. Programma Manager Medical Isotope Development
